Overcharging is one of the most difficult items in the current lithium battery safety test, so it is necessary to understand the mechanism of overcharging and the current measures to prevent overcharging.
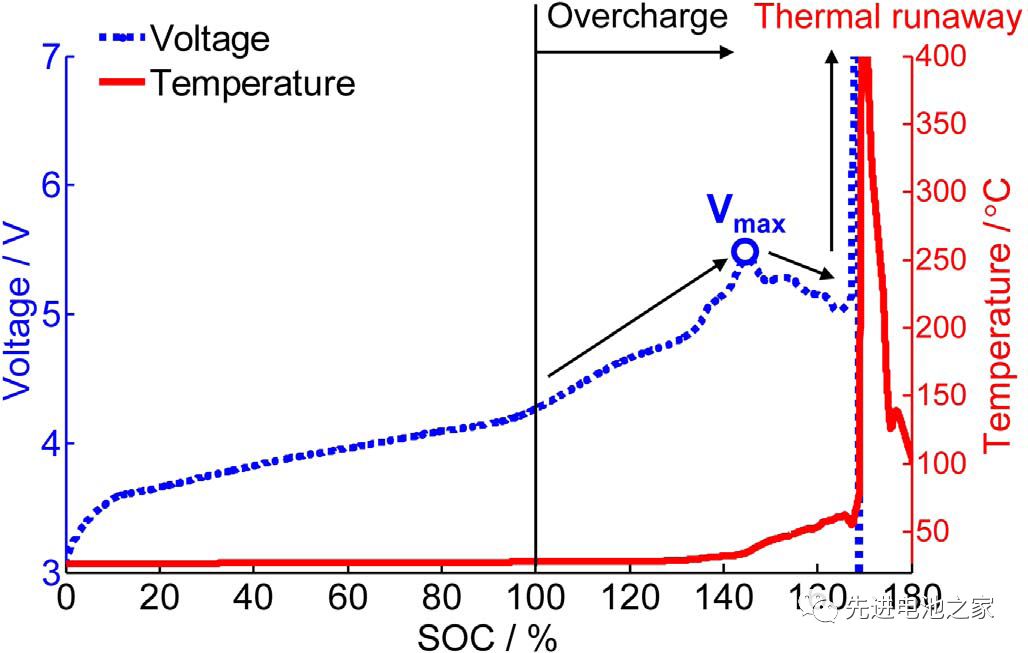
Picture 1 is the voltage and temperature curves of the NCM+LMO/Gr system battery when it is overcharged. The voltage reaches a maximum at 5.4V, and then the voltage drops, eventually causing thermal runaway. The voltage and temperature curves of the overcharge of the ternary battery are very similar to it.
When the lithium battery is overcharged, it will generate heat and gas. The heat includes ohmic heat and heat generated by side reactions, of which ohmic heat is the main one. The side reaction of the battery caused by overcharging is firstly that excess lithium is inserted into the negative electrode, and lithium dendrites will grow on the surface of the negative electrode (N/P ratio will affect the initial SOC of lithium dendrite growth). The second is that excess lithium is extracted from the positive electrode, causing the structure of the positive electrode to collapse, releasing heat and releasing oxygen. Oxygen will accelerate the decomposition of the electrolyte, the internal pressure of the battery will continue to rise, and the safety valve will open after a certain level. The contact of the active material with the air further generates more heat.
Studies have shown that reducing the amount of electrolyte will significantly reduce heat and gas production during overcharging. In addition, it has been studied that when the battery does not have a splint or the safety valve cannot be opened normally during overcharging, the battery is prone to explosion.
Slight overcharging will not cause thermal runaway, but will cause capacity fading. The study found that when the battery with NCM/LMO hybrid material as the positive electrode is overcharged, there is no obvious capacity decay when the SOC is lower than 120%, and the capacity decays significantly when the SOC is higher than 130%.
At present, there are roughly several ways to solve the overcharging problem:
1) The protection voltage is set in the BMS, usually the protection voltage is lower than the peak voltage during overcharging;
2) Improve the overcharge resistance of the battery through material modification (such as material coating);
3) Add anti-overcharge additives, such as redox pairs, to the electrolyte;
4) With the use of voltage-sensitive membrane, when the battery is overcharged, the membrane resistance is significantly reduced, which acts as a shunt;
5) OSD and CID designs are used in square aluminum shell batteries, which are currently common anti-overcharge designs. The pouch battery cannot achieve a similar design.
References
Energy Storage Materials 10 (2018) 246–267
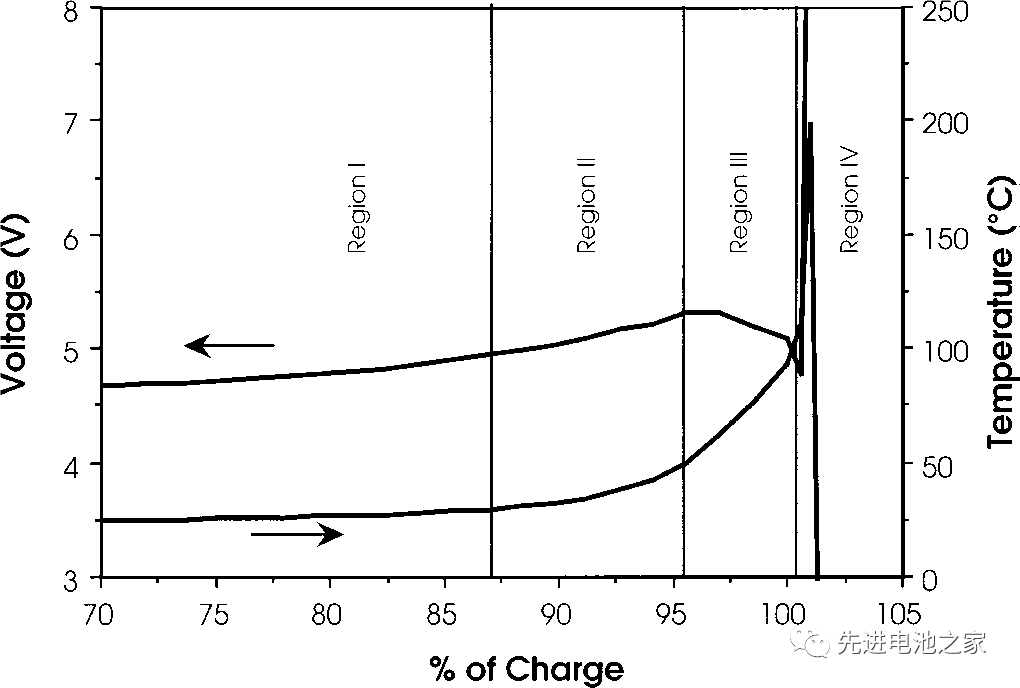
This time, we will introduce the voltage and temperature changes of the lithium cobalt oxide battery when it is overcharged. The picture below is the overcharge voltage and temperature curve of the lithium cobalt oxide battery, and the horizontal axis is the delithiation amount. The negative electrode is graphite, and the electrolyte solvent is EC/DMC. The battery capacity is 1.5Ah. The charging current is 1.5A, and the temperature is the internal temperature of the battery.
Zone I
1. The battery voltage rises slowly. The positive electrode of lithium cobalt oxide delithiates more than 60%, and metal lithium is precipitated on the negative electrode side.
2. The battery is bulging, which may be due to high-pressure oxidation of the electrolyte on the positive side.
3. The temperature is basically stable with a slight rise.
Zone II
1. The temperature starts to rise slowly.
2. In the range of 80~95%, the impedance of the positive electrode increases, and the internal resistance of the battery increases, but it decreases at 95%.
3. The battery voltage exceeds 5V and reaches the maximum.
Zone III
1. At about 95%, the battery temperature begins to rise rapidly.
2. From about 95%, until close to 100%, the battery voltage drops slightly.
3. When the internal temperature of the battery reaches about 100°C, the battery voltage drops sharply, which may be caused by the decrease of the internal resistance of the battery due to the increase in temperature.
Zone IV
1. When the internal temperature of the battery is higher than 135°C, the PE separator begins to melt, the internal resistance of the battery rises rapidly, the voltage reaches the upper limit (~12V), and the current drops to a lower value.
2. Between 10-12V, the battery voltage is unstable and the current fluctuates.
3. The internal temperature of the battery rises rapidly, and the temperature rises to 190-220°C before the battery ruptures.
4. The battery is broken.
The overcharging of ternary batteries is similar to that of lithium cobalt oxide batteries. When overcharging ternary batteries with square aluminum shells on the market, the OSD or CID will be activated when entering Zone III, and the current will be cut off to protect the battery from overcharging.
References
Journal of The Electrochemical Society, 148 (8) A838-A844 (2001)
Post time: Dec-07-2022